Aseptic Processing Equipment
For injectable, vaccine, and ampoule lines with complete automation and sterility.

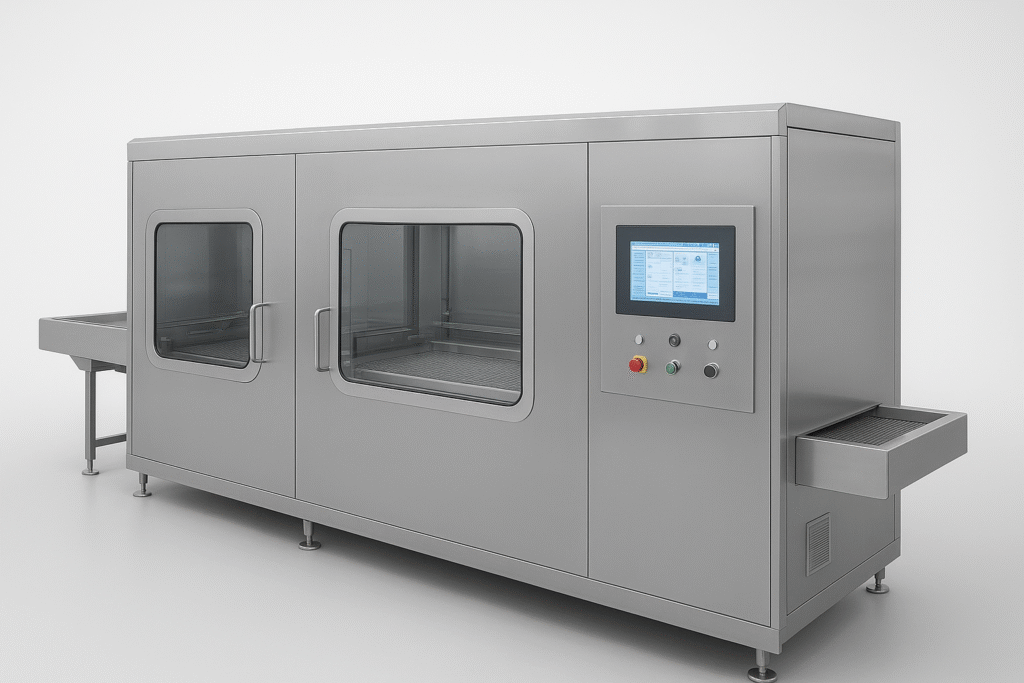
Our high-performance vial/ampoule washer ensures particulate-free containers prior to sterilization. With precision wash cycles, multiple water-air rinses, and minimal glass-to-glass contact, it safeguards container integrity while meeting GMP standards.
Key Features & Benefits :
✅Multi-Stage Washing – WFI and compressed air rinse.
✅ No Glass-to-Glass Contact – Reduces breakage risk.
✅ Automated Cycle Control – Consistent quality.
✅ 316L Stainless Steel – Hygienic, corrosion-resistant.
✅ Validation Ready – 21 CFR Part 11 compliant.
Applications :
Ideal for sterile injectable preparation lines
Key Features & Benefits :
✅ Continuous HEPA Airflow – ISO Class 5.
✅Validated Temperature Profile – Reliable endotoxin removal.
✅ Energy-Efficient Heating – Lower operational costs.
✅Full Automation – Seamless integration with washer and filler.
✅ Robust Build – 316L stainless steel.
Applications :
Sterile production lines for injectables, vaccines.

The modular filling, bunging, and sloppering station delivers precise dosing with minimal product loss, integrated with sterile component placement.
Key Features & Benefits :
✅High Fill Accuracy – Servo-driven systems.
✅ In-Line Stoppering – Controlled under laminar flow.
✅ Minimal Product Waste – Optimized design.
✅ Flexible Format Changes – Quick adjustments.
✅ CIP/SIP Ready – Easy cleaning and sterilization.
Applications :
Sterile liquids, vaccines, biotech formulations.
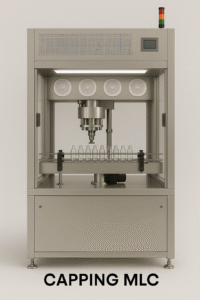
The capping unit secures aluminum caps onto vials with consistent torque, ensuring container closure integrity post-filling.
Key Features & Benefits :
✅ Consistent Torque Application – Reliable seal.
✅High Throughput – Matches upstream line speed.
✅ Compact Footprint – Space saving.
✅Minimal Maintenance – Rugged mechanics.
✅ GMP-Compliant Design – Smooth, cleanable surfaces.
Applications :
Sterile injectable packaging lines.

Key Features & Benefits :
✅High-Pressure Water Jets – Effective cleaning.
✅HEPA Drying – Particle-free finish.
✅ In-Line Operation – No process interruptions.
✅Water Recovery System – Sustainable operation.
✅Compact & Hygienic Build – Easy to clean.
Applications :
Pharma production for sterile injectables.
Key Features & Benefits :
✅ Servo-Controlled Label Placement – Accurate alignment.
✅Vision Inspection – Ensures correct label placement.
✅Quick Changeover – Minimal downtime.
✅Compact Footprint – Saves space.
✅ GMP-Grade Build – Easy cleaning.
Applications :
Sterile injectables, biotech products.


Key Features & Benefits :
✅Camera-Based Vision System – High detection accuracy.
✅360° Container Scanning – Complete coverage.
✅ Reject Mechanism – Automated removal of defective units.
✅Configurable Parameters – Matches product needs.
✅Data Logging – Supports quality assurance.
Applications :
Sterile production for parenteral drugs.

